What is Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) on Cosmetics - Microbiology - Microbiological limits about?
- What is Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) on Cosmetics - Microbiology - Microbiological limits in Vietnam about?
- What is the scope of application of the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) and what are the terms and definitions specified in this Vietnam Standard?
- What are the principles set out in Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014)?
- What are the microbiological limits in cosmetics specified in the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014)?
What is Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) on Cosmetics - Microbiology - Microbiological limits in Vietnam about?
The Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) is fully equivalent to ISO 17516: 2014.
Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) is compiled by the Institute for Drug Quality Control of Ho Chi Minh City, proposed by the Ministry of Health, appraised by the Directorate of Standards, Metrology and Quality, and announced by the Ministry of Science and Technology.
Every cosmetic manufacturer is responsible for microbiological safety and the quality of its products to ensure products are manufactured in good hygienic conditions. Although cosmetic products do not require sterility, they are not allowed to have too many microorganisms as well as specified microorganisms that have the potential to affect product quality or consumer safety. However, some cosmetic products considered low microbiological risk (TCVN 13641:2023 (ISO 29621)) may not need regular microbiological testing and manufacturers may decide not to test if they can ensure the product meets this standard.
Manufacturers should adhere to Good Manufacturing Practice (GMP) principles, ISO 22716, and must take necessary precautions to limit the entry of microorganisms from raw materials, processing and packaging.
The objective of this Vietnam standard is to develop acceptable levels of quantitative and qualitative microbiological limits for finished cosmetic products.
What is the scope of application of the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) and what are the terms and definitions specified in this Vietnam Standard?
Based on the provisions of Section 1 of the Vietnam Standard TCVN 13634: 2023, the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014) applies to all cosmetics and supports related units in assessing the microbiological quality of products. Microbiological testing does not need to be performed on products deemed to have a low risk of microbiological contamination based on the risk assessment described in TCVN 13641 (ISO 29621).
In the Vietnam Standard TCVN 13634: 2023 the following terms and definitions include:
Product
Defined cosmetic product fraction received in the laboratory for testing
Aerobic mesophilic microorganisms
Medium thermophilic bacteria, yeasts and molds, aerobic growth under conditions featured in TCVN 13633 (ISO 16212) and TCVN 13638 (ISO 21149).
Specified microorganisms
Medium-unwanted thermophilic bacteria or yeast contamination in cosmetic products is likely to cause bacterial contamination of the skin or eyes or is an indicator of lack of hygiene (TCVN 13635 (ISO 18415)).
Escherichia coli
Gram-negative, mobile, smooth bacilli (TCVN 12974 (ISO 21150)).
Pseudomonas aeruginosa
Gram-negative, mobile, smooth colonies with brown pigmentation or green luster (TCVN 16369 (ISO 22717)).
Staphylococcus aureus
Gram-positive cocci, often coalescing in clusters like bunches of grapes; smooth colonies usually have yellow pigmentation (TCVN 13640 (ISO 22718)).
Candida albicans
Yeast forms round, creamy, creamy colonies of white to ivory white on the surface of the selective medium (TCVN 13636 (ISO 18416)).

What are the principles set out in Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014)?
Under the provisions of Section 3 of the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014), the following principles are set:
The composition of raw materials and production conditions of cosmetics do not require sterility. However, microorganisms present in the product must not cause harmful effects on the safety of users or the quality of the product during the use of the product. Therefore, it is necessary to establish qualitative and/or quantitative microbiological limits for cosmetic products.
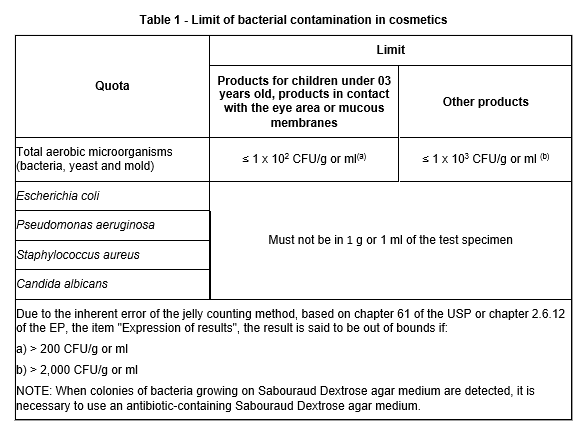
The allowable level for tropical areas is less than or equal to 1 x 103 CFU per gram or ml of product. However, note that the special allowance for specialized cosmetics for use in the eye area, for children under 3 years of age and on thin mucous membranes is less than or equal to 1 x 102 CFU per gram or ml per product. In addition, the interpretation of out-of-bounds results is considered by the inherent error of the lithic disk counting method (see Table 1).
At the same time, cosmetics must be free of E. coli, S. aureus, P. aeruginosa, and C. albicans in 1 g or 1 ml of the product.
This standard specifies microbiological limits for cosmetics. Where necessary, test methods by international standards (annex A) shall be used to assess conformity to the quality level of this standard.
What are the microbiological limits in cosmetics specified in the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014)?
Under the provisions of Section 4 of the Vietnam Standard TCVN 13634: 2023 (ISO 17516: 2014), the microbiological limits in cosmetics are specified as follows:
To ensure the quality of the product and safety for users, the total number of microorganisms capable of recovering from the product must remain stable or diminished over its lifetime. The presence of microorganisms is allowed provided that they are not capable of proliferation in the product. This may be based on a risk assessment, including assessments of preservative efficacy (TCVN 13632 (ISO 11930)) or demonstration that the product cannot promote microbiological growth (TCVN 13641 (ISO 29621)).
Based on these viewpoints, the microbiological limits mentioned in the following Table 1 shall be applied:
LawNet