What is the form of technical document describing the type of imported medical device for issuance of import license in Vietnam?
- What is the form of technical document describing the type of imported medical device for issuance of import license in Vietnam under Circular 10/2023/TT-BYT?
- What are the contents of technical document describing the type of imported medical device for issuance of import license in Vietnam?
- What is the application for the import license of medical device in Vietnam?
What is the form of technical document describing the type of imported medical device for issuance of import license in Vietnam under Circular 10/2023/TT-BYT?
Pursuant to Form No. 06, Annex VII issued together with Circular 10/2023/TT-BYT, stipulating the form of technical document describing the type of imported medical device for issuance of import license as follows: :
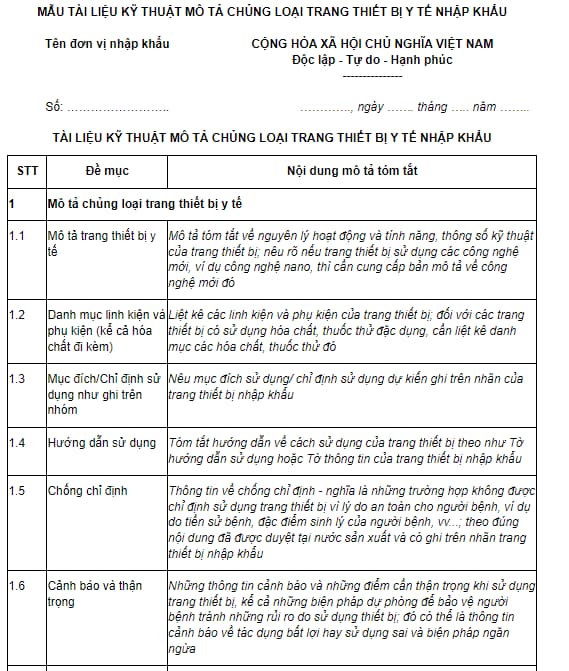
Download form of technical document describing the type of imported medical device for issuance of import license: here
What is the form of technical document describing the type of imported medical device for issuance of import license in Vietnam? (Image from the Internet)
What are the contents of technical document describing the type of imported medical device for issuance of import license in Vietnam?
Pursuant to Form No. 06, Appendix VII issued together with Circular 10/2023/TT-BYT, stipulating technical document describing the type of imported medical device for issuance of import license with the following contents:
- Description of the type of medical device
+ Description of medical device: Brief description of operating principles and features, technical parameters of the equipment; state if the equipment uses new technology, provide a description of the new technology.
+ List of components and accessories (including accompanying chemicals):
List equipment components and accessories; for equipment using special-use chemicals and reagents, a list of such chemicals and reagents should be listed.
+ Purpose/Indication for use as stated on the group: Indicate the intended use/indication of use on the label of the imported equipment.
+ Instructions for use: Summary of instructions on how to use the equipment such as the User's Manual or the Information Sheet of the imported equipment.
+ Contraindications: Information on contraindications - i.e. situations where the use of the device is not indicated for the safety of the patient, for example due to medical history, physiological characteristics of the disease, etc. …; in accordance with the content approved in the country of manufacture and stated on the label of imported equipment
+ Warnings and cautions: Warnings and cautions when using the device, including precautions to protect patients from risks caused by using the device; It could be warning information about adverse effects or misuse and precautions.
+ Possible Adverse Effects: Information on adverse events related to the use of the medical device was recorded through clinical trials and post-marketing follow-up performed previously for the site. Imported medical device.
- Information on products that have been circulated in countries (if any): Provide information about countries that have approved to allow the circulation of products, the first country to grant registration/permit for circulation of medical device.
- Indications already registered in other countries (if any): List the countries where marketing registration has been issued with the indication for use approved in that country; date of registration.
- Information about the safety/remarkable operation of medical device products:
+ Provide information on the number of reported adverse reactions related to the use of the device; post-marketing recall/adjustment measures have been implemented at the request of the regulatory authorities of the countries.
+ If the equipment contains one of the following components, it is necessary to provide information about:
++ Human or animal cells or tissues or their derivatives intended for use in a non-viable form - for example, artificial heart valves of pig origin, cat intestines...;
++ Cells, tissues and/or derivatives of microbial or recombinant origin - eg hyaluronic acid-based dermal fillers obtained by microbial fermentation...; Contains irritating, ionizing ingredients - eg X-ray; or non-ionizing - E.g. laser, ultrasound, etc.
What is the application for the import license of medical device in Vietnam?
Pursuant to the provisions of Clause 2, Article 48 of Decree 98/2021/ND-CP (added by point b, Clause 12, Article 1 of Decree 07/2023/ND-CP) the following regulations:
Import license
....
2. An application for the import license consists of:
a) The application form for import license;
b) A synopsis of the technical description of the medical device in Vietnamese;
c) Certificate of conformity with quality control standards of the manufacturer bearing the applicant’s certification;
d) If the medical device is imported to serve research: a certified true copy of the decision to approve the research and documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
dd) If the medical device is imported to serve training purposes: the original copy of the training program and documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
e) If the medical device is imported to serve testing, inspection, experiment, or performance evaluation: the certification indicating the quantity of the imported device given by the agency competent to carry out such testing, inspection, experiment, or performance evaluation;
g) If the medical device is imported as aid: a copy of the decision to approve the aid and documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
h) If the medical device is imported as gift or present given to a health facility: the original copy of the training program and documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
i) If the medical device is imported to serve charitable medical examination and treatment: documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
k) If the medical device is imported to serve a health facility’s special diagnosis demand: documents bearing the applicant’s certification proving that the device has been granted marketing authorization by a competent authority;
l) If the medical device is imported to serve personal treatment of illness, including personalized medical devices: a copy of the physician’s prescription which is consistent with the applicant’s illness;
m) If the medical device is imported to serve a trade fair, exhibition, display or product launch event: copies of documents on the program, invitation letter and service contract;
n) If the medical device is imported to serve the purposes of epidemic prevention and control or disaster recovery, the following documents are required:
- A competent authority’s approval for import of the medical device to serve epidemic prevention and control or disaster recovery;
- Documents bearing the applicant’s certification proving that the device has been granted marketing authorization or license for emergency use by a competent authority.
o) In the case specified in Point e Clause 1 of this Article, the application for import license shall include:
- The original copies or certified true copies of the decision to approve the investment guidelines and the investment decision for an investment project or the decision to approve project documents for a project on technical assistance, project costs or grants other than ODA grants, in which the import of medical devices must be indicated;
- The original copy of certified true copy of the contract for supply of medical devices for the project;
- The power of attorney granted by the product owner to the applicant which must be still valid at the date of application submission. Either the document bearing consular legalization or the certified true copy thereof is accepted;
- The certificate of eligibility to provide warranty services granted by the product owner, except disposable medical devices defined by product owners or cases where there are documents proving that the medical device is not under warranty. Either the document bearing consular legalization or the certified true copy thereof is accepted;
- The unexpired CFS (for imported medical devices). Either the document bearing consular legalization or the certified true copy thereof is accepted. If the CFS is made neither in English nor in Vietnamese, it shall be translated into Vietnamese. The Vietnamese translation must be certified as prescribed by law.
Accordingly, the application for the import license of medical device must contain the documents specified above.
LawNet