What is the application form for a pharmacy practice certificate in Vietnam?
What is the application form for a pharmacy practice certificate in Vietnam?
The form of application for a pharmacy practice certificate according to current regulations is form No. 02, Appendix I issued together with Decree 54/2017/ND-CP. Below is a picture of the application form for a pharmacy practice certificate:
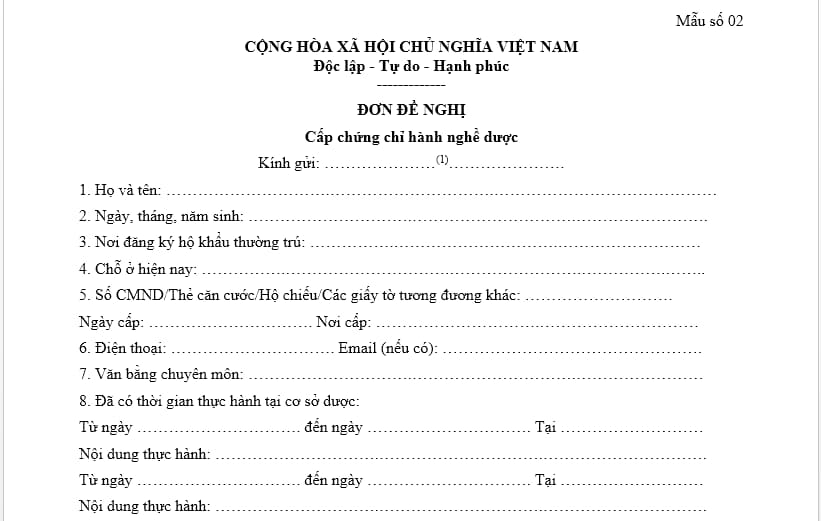
Download the application form for a pharmacy practice certificate: Click here.

What is the application form for a pharmacy practice certificate in Vietnam? (Image from the Internet)
What are the instructions for filling out the application form for a pharmacy practice certificate in Vietnam?
Pursuant to Form 02, Appendix I issued together with Decree 54/2017/ND-CP, guiding the filling of the application form for a pharmacy practice certificate as follows:
- (1) Name of the agency issuing the People's Republic of China.
- (2) Place name.
- (3) Practice positions as prescribed from Article 15 to Article 22 of the Law on Pharmacy, specifically:
+ The person in charge of pharmacy expertise of the drug-manufacturing establishment, except for cases 2 and 3 below.
+ The person in charge of pharmacy expertise of the manufacturer of medicinal ingredients is active ingredients, excipients, capsule shells.
+ The person in charge of pharmacy expertise of the establishment of manufacturing vaccines, biological products and raw materials for the production of vaccines and biological products.
+ The person in charge of quality assurance of the drug-manufacturing establishment, except for cases 5 and 6 below.
+ The person in charge of quality assurance of vaccine and biological product manufacturers.
+ The persons in charge of quality assurance of establishments producing medicinal ingredients are active ingredients, excipients, and capsule shells.
+ The person in charge of pharmacy expertise, the person in charge of quality assurance of herbal ingredient manufacturers;
+ The person in charge of pharmacy expertise, the person in charge of quality assurance of business households and cooperatives producing herbal ingredients.
+ The person in charge of the professional pharmacy of the establishment that wholesales drugs and medicinal ingredients, except for cases 10 and 11 below.
+ The person in charge of pharmacy expertise of a vaccine or biological product wholesaler.
+ The person in charge of pharmacy expertise of a wholesaler of herbal ingredients, herbal drugs or traditional drugs.
+ The person in charge of pharmacy expertise of establishments exporting or importing drugs or medicinal ingredients, except for the following cases 13 and 14.
+ The person in charge of pharmacy expertise of the establishment of exporting or importing vaccines and biological products.
+ The person in charge of pharmacy expertise of the exporter or importer of herbal ingredients, herbal drugs or traditional drugs.
+ The person in charge of pharmacy expertise of the pharmacy.
+ The person in charge of pharmacy expertise of the drugstore.
+ The person in charge of pharmacy expertise of the commune health station's medicine cabinet
+ The person in charge of pharmacy expertise of establishments specializing in retailing of herbal ingredients, herbal drugs, and traditional drugs.
+ The person in charge of pharmacy expertise of establishments providing testing services for drugs and medicinal ingredients, except for case 20 below.
+ The person in charge of pharmacy expertise of establishments providing testing services for vaccines, biological products,
+ The person in charge of pharmacy expertise of establishments providing clinical trial services and bioequivalence testing of drugs, except for case 22 below.
+ The person in charge of professional expertise of establishments providing clinical trial services or bioequivalence testing of herbal drugs or traditional drugs.
+ The person in charge of clinical pharmacy work of medical examination and treatment establishments, except for case 24 below.
+ The person in charge of clinical pharmacy work of traditional medicine examination and treatment establishments.
+ The person in charge of pharmacy expertise of the establishment providing the service of preserving drugs and medicinal ingredients, except for case 26 below.
+ The person in charge of the professional pharmacy of the establishment providing the service of preserving vaccines and biological products.
What is the application for a pharmacy practice certificate in Vietnam?
Pursuant to Clause 1, Article 3 of Decree 54/2017/ND-CP (Point a, Clause 1, Article 3 of Decree 54/2017/ND-CP as amended by Clause 2, Article 5 of Decree 155/2018/ND-CP, Points c and g, Clause 1, Article 3 of Decree 54/2017/ND-CP annulled by Clause 1, Article 4 of Decree 155/2018/ND-CP) stipulate that an application file for a pharmacy practice certificate includes:
- An application for a pharmacy practice certificate is made according to Form No. 02 in Appendix I issued with Decree 54/2017/ND-CP.
- Certified copy of professional diploma. For diplomas issued by foreign training institutions, they must be accompanied by a certified true copy of the equivalence recognition certificate issued by the agency competent to recognize equivalence as prescribed in Clause 2, Article 18 of Decree 54/2017/ND-CP.
- The original or certified true copy of the certificate of practice as prescribed in Form No. 03 in Appendix I issued with Decree 54/2017/ND-CP. In case of practicing at many establishments, the practice time is counted as the total practice time at the establishments, but a certificate of practice time of each establishment is required.
- In case the application for a pharmacy practice certificate has different scopes of activities and requires different practice periods and professional practice establishments, the application must contain a certificate of professional practice time and contents of the application. content of professional practice of one or several establishments that meet the requirements of each scope and position of practice.
In case the scope of professional activities has the same requirements on practice time and professional practice facilities, a separate certification is not required for each scope of professional activities;
- The original or a certified true copy of the certificate of examination results issued by the examination organization specified in Clause 2, Article 28 of Decree 54/2017/ND-CP, in case the pharmacy practice certificate is issued in the form of a pharmacy practice certificate.
LawNet