Vietnam: What are the regulations on the list of medicinal chemicals and bio-products covered by health insurance participants?
What is the list of medicinal chemicals and bio-products covered by health insurance participants in Vietnam?
The list of pharmaceutical and bio-chemical drugs within the scope of entitlement of health insurance participants is issued together with Circular 20/2022/TT-BYT
Pursuant to Appendix I issued together with Circular 20/2022/TT-BYT, the list of pharmaceutical and bio-chemical drugs within the scope of entitlement of health insurance participants is prescribed as follows:
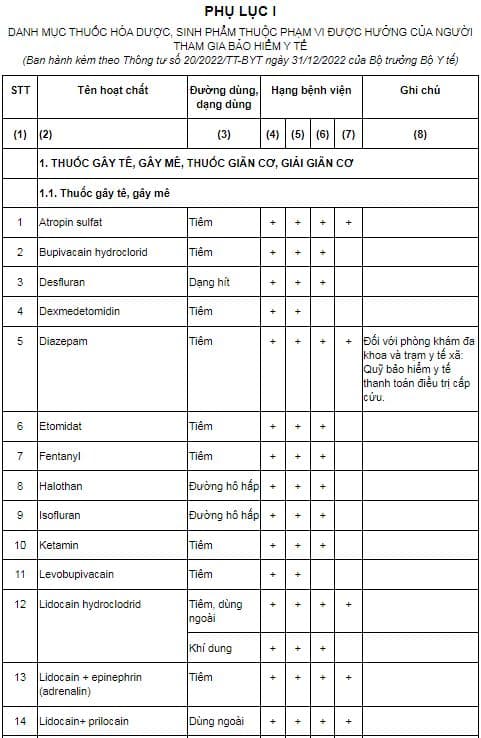
Download the list of pharmaceutical chemicals and bio-products covered by health insurance participants: Here 
Vietnam: What are the regulations on the list of medicinal chemicals and bio-products covered by health insurance participants?
How to read the list of pharmaceutical and biochemical drugs covered by health insurance participants in Vietnam?
Pursuant to Article 2 of Circular 20/2022/TT-BYT stipulating the structure of the list of drugs and classification of use as follows:
- The list of pharmaceutical and biochemical drugs in Appendix I issued together with Circular 20/2022/TT-BYT is arranged into groups according to therapeutic effects classified into 08 (eight) columns, specifically as follows:
+ Column 1: Write the sequence number of the drug in the List
+ Column 2: Write the name of the drug. The drug name is inscribed after the active ingredient by the International Non-proprietary Name (INN). In case there is no international common name, it shall be written according to the name of the active ingredient licensed for circulation or the name in the diagnosis and treatment guidelines of the Ministry of Health. The drugs are arranged in large groups, according to the code anatomy, treatment, chemistry (ATC)
+ Column 3: Record the route of administration, use (usage) of the drug; do not write the content; do not specify the dosage form, except for some different forms of preparation. clear of potency, and therapeutic effect. Routes of administration, forms of administration (usage) of drugs in the List of pharmaceutical chemicals and bio-products are understood and agreed as follows:
++ Oral administration includes oral medications, chewing, lozenges;
++ Injections include intramuscular, subcutaneous, intracutaneous, intravenous, intraglacial injection, intraocular injection, intraocular injection in the menstrual fluid of the eye, injection or infusion into the cavities of the body;
++ External use includes topical drugs, skin rubbing, skin paste, skin spray, wash, applied to mucous membranes;
++ Placement includes vaginal suppositories, insertion, enema, direct, sublingual placement;
++ Respiration includes purulent sprays, inhaled forms (solutions, suspensions, powders used to sing), aerosols
++ Eye drops include eye drops, eye exams; ear drops include ear drops; nasal drops include nasal drops, nasal sprays;
+ Routes of administration and other forms of administration are specified in the List for some drugs with routes of administration and special use;
+ Columns 4, 5, 6, 7: Record the class of the hospital used and pay for health insurance. Drugs and active ingredients on the list of drugs specified in Appendix I are used and paid for health insurance at medical examination and treatment establishments according to hospital class, specifically as follows:
++ Special and Grade I hospitals use the drugs specified in column 4;
++ Grade II hospitals use the drugs specified in column 5
General principles of drug payment for patients participating in health insurance
++ Grade III and Class IV hospitals, including polyclinics belonging to general hospitals or health centers of districts, towns and cities directly under provinces and centrally-run cities, polyclinics, specialized clinics, private midwives that have been used by competent state officials on the equivalent of Line III. drugs specified in column 6;
++ Polyclinics, specialized clinics, private midwives that have not been assigned technical expertise; commune, ward, township, health agency and equivalent health stations (hereinafter collectively referred to as commune health stations) using the drugs specified in column 7;
+ Column 8: Record conditions, payment rates and record specific owners of some drugs.
Under what circumstances will the health insurance fund not pay in Vietnam?
Pursuant to Clause 3 Article 3 of Circular 20/2022/TT-BYT stipulating the general principle of payment of drug costs for patients participating in health insurance as follows:
- Drugs and batches of drugs that have been decided to suspend circulation or recall. The non-payment of drugs or batches of drugs that have been decided to suspend circulation and recall according to the scope of application in the written notice or decision on the suspension of circulation or recall of such drugs or batches of drugs by the Ministry of Health;
- The cost of drugs has been factored into the price of technical services, medical examinations, treatment bed dates or package prices according to current regulations;
- The cost of drugs is covered by the state budget or other funding sources;
- Drugs used in clinical trials, scientific research.
Circular 20/2022/TT-BYT will come into force from March 01, 2023.
LawNet
