Vietnam’s Circular No. 30/2015/TT-BYT: What is medical equipment?
Circular No. 30/2015/TT-BYT on import of medical equipment was issued by the Minister of Health of Vietnam on October 12, 2015.
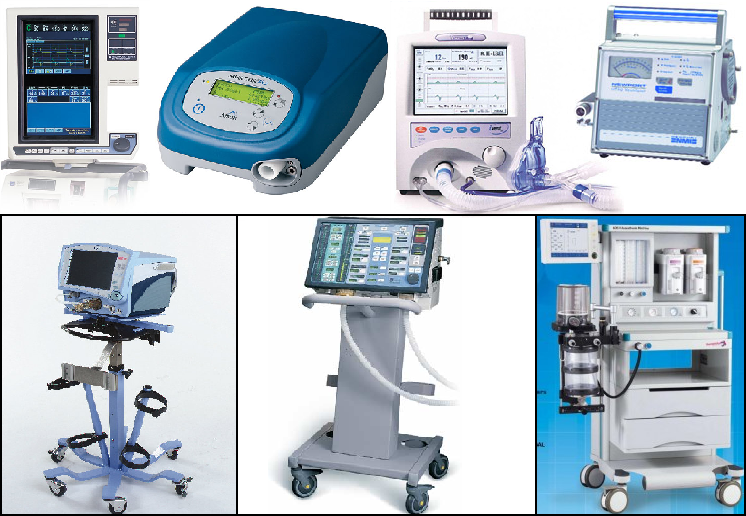
According to Circular No. 30/2015/TT-BYT of the Ministry of Health of Vietnam, medical equipment is the types of equipment, tool, material and in-vitro diagnosis chemical and software used separately or combined with each other as indicated by the owner to serve people for one or a lot of purposes as follows:
- Diagnosis, prevention, monitoring, treatment and mitigation of disease or injury compensation;
- Checking, replacement, modification or surgery support and physiological process;
- Life support or sustainment;
- Conception control;
- Sterilization of medical equipment (not including chemicals and insecticides and disinfectants for domestic and medical use);
- Use for medical equipment;
- Special transport for medical activities.
Concurrently, the import of medical equipment in the form of foreign aid (including medical equipment of over 80% quality), temporary import and re-export, temporary export and re-import, border transfer of goods, medical equipment as movable property, in service of personal needs with diplomatic status and personal luggage shall comply with the provisions of Decree No. 187/2013/ND-CP dated 20/11/2013 of Vietnam’s Government detailing the implementation of the Commercial Law on trading of international goods and activities of trading and processing agents and goods transit with foreign countries and the guidelines of the Ministry of Industry and Trade.
Besides, Circular No. 30/2015/TT-BYT also stipulates that medical equipment owner (hereafter referred to as owner) is any organization or individual directly implementing or permitting other organizations or individuals to use his/her name to provide the medical equipment in his/her own name or any label, design, commercial name or other names or other codes under the ownership or control of such organizations or individuals and shall take responsibility for the design, production, assembly, label, packaging, or maintenance, repair of medical equipment or define a purpose of use for such medical equipment.
View more details at Circular No. 30/2015/TT-BYT of the Ministry of Health of Vietnam, effective from November 30, 2015.
Le Hai
- Number of deputy directors of departments in Vietnam in accordance with Decree 45/2025/ND-CP
- Cases ineligible for pardon in Vietnam in 2025
- Decree 50/2025 amending Decree 151/2017 on the management of public assets in Vietnam
- Circular 07/2025 amending Circular 02/2022 on the Law on Environmental Protection in Vietnam
- Adjustment to the organizational structure of the Ministry of Health of Vietnam: Certain agencies are no longer listed in the organizational structure
- Vietnam aims to welcome 22-23 million international tourists in Vietnam in 2025
-

- Guidelines on direct payment of medication and ...
- 16:00, 15/01/2025
-
- Modification of transitional provisions regarding ...
- 15:35, 13/01/2025
-

- Financial support levels for purchasing and repairing ...
- 09:00, 23/11/2024
-
- Detailed economic - technical characteristics ...
- 09:00, 13/11/2024
-

- List of medical equipment assigned HS codes according ...
- 10:47, 23/10/2024
-

- Notable new policies of Vietnam effective as of ...
- 16:26, 11/04/2025
-
.Medium.png)
- Notable documents of Vietnam in the previous week ...
- 16:21, 11/04/2025
-
.Medium.png)
- Notable documents of Vietnam in the previous week ...
- 16:11, 02/04/2025
-
.Medium.png)
- Notable new policies of Vietnam to be effective ...
- 16:04, 02/04/2025
-
.Medium.png)
- Notable new policies of Vietnam effective from ...
- 14:51, 21/03/2025
 Article table of contents
Article table of contents
