Vietnam: What are the sample 1st mid-semester question papers of 11th-grade Chemistry under the Knowledge Connection Curriculum? What does the 11th-grade Chemistry curriculum cover?
What are the sample 1st mid-semester question papers of 11th-grade Chemistry under the Knowledge Connection Curriculum in Vietnam? What does the 11th-grade Chemistry curriculum cover?
Below is the sample 1st mid-semester question papers of 11th-grade Chemistry under the Knowledge Connection Curriculum in Vietnam:
1st mid-semester question papers - Knowledge Connection
Subject: 11th-grade Chemistry
Part I: Multiple Choice
Question 1. Which of the following statements is correct?
A. In a one-way reaction, product substances can react with each other to form reactants.
B. In a reversible reaction, product substances cannot react with each other to form reactants.
C. A one-way reaction is a reaction that always occurs incompletely.
D. A reversible reaction is a reaction that occurs in two opposite directions under the same conditions.
Question 2. When at equilibrium, which of the following statements is correct?
A. The rate of the forward reaction is less than the rate of the reverse reaction.
C. The concentration of products is always greater than that of reactants.
D. The concentration of the substances does not change.
Question 3. Dissociation is
A. the process of breaking down substances into new substances when dissolved in water.
B. the process of combining ions into molecules in a solution.
C. CuCl2.
D. NaOH.
Question 5. The pH value of a solution is calculated by which of the following expressions?
A. pH = −log[H+].
B. pH = 14 + log[H+].
C. pH = 14 −log[OH-].
D. pH = log[OH−].
Question 6. The gastric juices in a normal human have a pH range of 1.5 – 3.5. The environment of gastric juices is
A. a basic environment.
B. an acidic environment.
C. a neutral environment.
D. a neutralized environment.
Question 7. According to the Bronsted – Lowry acid-base theory, a base is
A. an electron acceptor.
B. an electron donor.
C. a proton acceptor.
D. a proton donor.
Question 8. In nature, in which of the following forms does the element nitrogen (N) exist?
A. Exists in both elemental and compound forms.
B. Exists only in elemental form.
C. Exists only in organic compound form.
D. Exists only in inorganic compound form.
Question 9. The structural characteristic of an N2 molecule is
A. has one triple bond.
B. has one double bond.
C. has two double bonds.
D. has two triple bonds.
Question 10. The geometric shape of an ammonia molecule is
A. equilateral triangle.
B. regular tetrahedron.
C. straight line.
D. triangular pyramid.
Question 11. When a few drops of phenolphthalein are added to an NH3 solution, the solution turns
A. pink.
B. yellow.
C. red.
D. blue.
Question 12. Which of the following salts is highly soluble in water?
A. CaCO3.
B. BaSO4.
C. NH4Cl.
D. AgCl.
Question 13. When NaOH solution is added to NH4Cl solution and heated, the gas that is released is
A. a light green gas.
B. a colorless gas with a pungent odor that turns moist litmus paper blue.
C. a brownish-red gas that turns moist litmus paper blue.
D. a colorless, odorless gas.
Question 14. Which group of metals does not react with HNO3?
A. Al, Cu.
B. Au, Pt.
C. Mg, Au.
D. Ag, Pt.
Question 15. Pure HNO3 is a colorless liquid, but HNO3 solution that is left for a long time usually turns yellow due to
A. HNO3 being highly soluble in water.
B. HNO3 solution being a strong acid.
C. HNO3 solution having strong oxidizing properties.
D. Pure HNO3 being unstable, partially decomposing to release NO2.
Question 16. Which of the following is not a structural characteristic of the nitric acid molecule?
A. The nitrogen atom has an oxidation state of +5, which is the highest oxidation state of nitrogen.
B. The O – H bond is highly polar towards the oxygen atom.
C. The N → O bond is an ionic bond.
D. The N → O bond is a donor-acceptor bond.
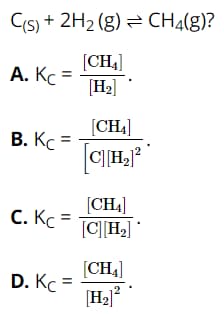
Question 17. Which of the following expressions is the equilibrium constant expression (Kc) for the reaction:
Question 18. Consider the chemical equilibria:
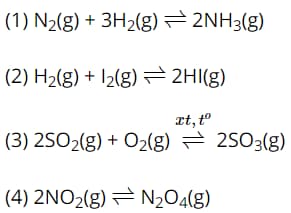
When the pressure is changed, the number of chemical equilibria that are shifted is
A. 1.
B. 2.
C. 3.
D. 4.
Question 19. For the following equilibrium (in a closed container):
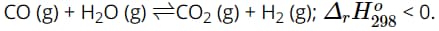
The sequence of factors: (1) Increase temperature; (2) Add some water vapor; (3) Add some H2; (4) Increase the overall pressure of the system; (5) Use a catalyst.
The set of factors that change the equilibrium of the system includes:
A. (1), (4), (5).
B. (1), (2), (3).
C. (2), (3), (4).
D. (1), (2), (4).
Question 20. Which of the following dissociation equations is incorrect?
A. HBr → H^+^ + Br^−^.
B. HCOOH ⇌⇌ HCOO^−^ + H^+^.
C. 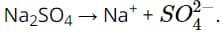
D. 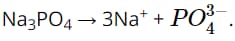
Part II: Essay
Question 1: In a CoCl2 (pink) salt solution, there is the following chemical equilibrium:
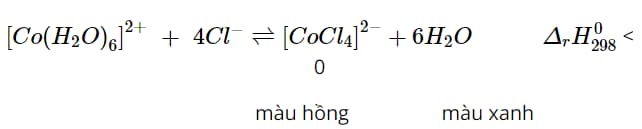
Predict the color change of the test tube containing the CoCl2 solution in the following cases:
a) Slowly add concentrated HCl.
b) Immerse the test tube in hot water.
Question 2: Determine if a solution of iron alum (NH4Fe(SO4)2.12H2O) is acidic or basic. Why can iron alum be used to remove suspended particles in water?
Question 3: A gas mixture X consists of N2 and H2 with a density relative to H2 of 3.6. When X is heated in a closed container with Fe catalyst, a gas mixture Y is obtained with the number of moles reduced by 8% compared to the initial amount. Determine the efficiency of the NH3 synthesis reaction.
-------End-------
Note: The above sample 1st mid-semester question papers of 11th-grade Chemistry under the Knowledge Connection Curriculum in Vietnam is for reference only.
Students should combine revision with the 1st mid-semester question papers given by teachers to have the best results.
What are the sample 1st mid-semester question papers of 11th-grade Chemistry under the Knowledge Connection Curriculum in Vietnam? What does the 11th-grade Chemistry curriculum cover? (Picture from the Internet)
What does the 11th-grade Chemistry curriculum in Vietnam cover?
According to Subsection 1, Section V of the general education program for Chemistry issued with Circular 32/2018/TT-BGDDT, the 11th-grade Chemistry curriculum in Vietnam includes:
Basic Chemistry Knowledge
- Chemical Equilibrium
Inorganic Chemistry
- Nitrogen and Sulfur
Organic Chemistry
- General Introduction to Organic Chemistry
- Hydrocarbons
- Halogen Derivatives - Alcohols - Phenols
- Carbonyl Compounds (Aldehydes - Ketones) - Carboxylic Acids
What are the objectives of the general education program for 11th-grade Chemistry in Vietnam?
By Section III of the general education program for Chemistry issued with Circular 32/2018/TT-BGDDT, the objectives of the general education program for Chemistry in Vietnam are defined as follows:
- The Chemistry subject develops students' chemistry competence and contributes to forming and developing key qualities and general competencies, especially the scientific worldview, through coordination with other subjects and educational activities; instilling a passion for learning and research; honesty; a respectful attitude towards natural laws, interaction with nature that aligns with sustainable development requirements; the ability to choose a career that matches one's abilities, interests, and personal conditions.

