Vietnam: What are the 1st mid-semester question papers of 10th-grade Chemistry and answers thereof? What is the teaching equipment for 10th-grade Chemistry?
What are the 1st mid-semester question papers of 10th-grade Chemistry and answers thereof in Vietnam?
Below are the 1st mid-semester question papers of 10th-grade Chemistry and the answers thereof:
1st mid-semester question paper - Knowledge Connection
2024 - 2025 School Year
Subject: 10th-grade Chemistry
Time: ... minutes
(excluding the time to distribute the question papers)
Part 1: Multiple Choice (7 points)
Question 1: The subject of research in chemistry is
A. the orbit of Earth's motion.
B. the speed of light in a vacuum.
C. the evolution of humans.
D. the transformation of substances.
Question 2: A type of hydrogen atom has the simplest structure, consisting of only 1 electron and 1 proton (not containing a neutron). Which statement is correct about this hydrogen atom?
A. This is the heaviest atom among known atoms so far.
B. The atomic mass is approximately 2 amu.
C. The nucleus of the atom is about 1818 times heavier than the shell.
D. The size of the atom is equivalent to the size of its nucleus.
Question 3: An atom consists of
A. a nucleus containing protons, neutrons, and an atomic shell containing electrons.
B. a nucleus containing protons, electrons.
C. a nucleus containing protons, electrons, and an atomic shell containing neutrons.
D. a nucleus and an atomic shell containing protons.
Question 4: The nucleus of atom X contains 15 protons and 16 neutrons. The mass number of the nucleus of atom X is
A. 30. B. 31. C. 32. D. 46.
Question 5: Given the following atoms: A (Z = 8, A = 16), B (Z = 9, A = 19), C (Z = 8, A = 17), D (Z = 7, A = 17). Among these atoms, which atoms belong to the same chemical element?
A. Atom A and atom B.
B. Atom C and atom D.
C. Atom A and atom C.
D. Atom B and atom C.
Question 6: Nitrogen atom has 7 electrons. The nuclear charge of this atom is
A. +7. B. -7. C. 7+. D. 7.
Question 7: An atom contains 11 electrons and 12 neutrons. The symbol for this atom is
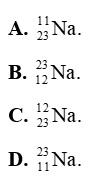
Question 8: Naturally occurring lithium has 2 isotopes: 7Li and 6Li. The average atomic mass of Li is 6.93. The percentage of isotope 7Li atoms is
A. 93%. B. 7%. C. 78%. D. 22%.
Question 9: The image below indicates the shape of which orbital?
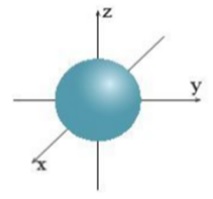
A. Orbital s.
B. Orbital p.
C. Orbital d.
D. Orbital f.
Question 10: The distribution of electrons into shells and subshells is based on
A. mass number.
B. nuclear charge.
C. atomic mass.
D. electron energy levels.
Question 11: The maximum number of electrons in the M shell is
A. 3. B. 6. C. 9. D. 18.
Question 12: The fourth electron shell is also known as
A. K shell.
B. M shell.
C. N shell.
D. L shell.
Question 13: The electron configuration of sulfur atom (Z = 16) is

Question 14: In the ground state, the number of unpaired electrons in phosphorus atom (Z = 15) is
A. 1. B. 2. C. 3. D. 4.
Question 15: In the ground state, the outer electron configuration of atom X is 3s^2^. The atomic number of element X is
A. 12. B. 13. C. 11. D. 14.
Question 16: The atomic number of a chemical element equals
A. the ordinal number of the group.
B. the ordinal number of the period.
C. the ordinal number of the element box.
D. the number of outer shell electrons.
Question 17: The electron configuration of oxygen atom is 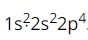 . The position of oxygen in the periodic table is
. The position of oxygen in the periodic table is
A. box 6, period 2, group VIA.
B. box 6, period 3, group VIB.
C. box 8, period 2, group VIB.
D. box 8, period 2, group VIA.
Question 18: The element aluminium (Al) has an atomic number of 13. Al belongs to which element block
A. s. B. p. C. d. D. f.
Question 19: Which element's atom has the strongest non-metallic character?
A. Fluorine.
B. Bromine.
C. Phosphorus.
D. Iodine.
Question 20: Which statement about the trend of metallic properties in the periodic table of chemical elements is correct?
A. The metallic properties of elements increase moving left to right in a period and top to bottom in a group.
B. The metallic properties decrease moving left to right in a period and increase top to bottom in a group.
C. The metallic properties decrease moving left to right in a period and top to bottom in a group.
D. The metallic properties increase moving left to right in a period and decrease top to bottom in a group.
Question 21: Electronegativity is
A. a measure characterizing the ability to donate electrons of an element's atom when forming chemical bonds.
B. a measure characterizing the ability to form molecules.
C. a measure characterizing the ability to form atoms.
D. a measure characterizing the ability to attract electrons of an element's atom when forming chemical bonds.
Question 22: Sulfur belongs to group VIA of the periodic table. The highest oxide formula of sulfur is
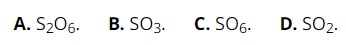
Question 23: Which of the following oxides has the strongest basic property?
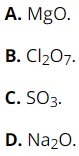
Question 24: Among the following substances, which one has the weakest acidic property?
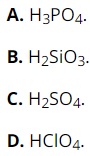
Question 25: The metal elements of group IA are also called
A. alkali metals.
B. alkaline earth metals.
C. halogens.
D. noble gases.
Question 26: Element M belongs to period 3, group VA of the periodic table. The atomic number of element M is
A. 16. B. 14. C. 15. D. 13.
Question 27: The outermost electron configuration of element X is 3s1. The highest oxide of X possesses which of the following properties?
A. Metallic property.
B. Non-metallic property.
C. Acidic property.
D. Basic property.
Question 28: Arrange the basicity of NaOH, Mg(OH)2, Al(OH)3 in decreasing order as
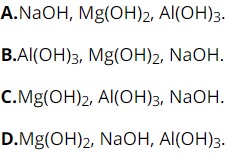
Part 2: Essay (3 points)
Question 1 (1 point): Two elements A and B are consecutive in the same period of the periodic table and have a total nuclear charge of 25. Determine the position (box, period, group) of these two elements in the periodic table (provide a brief explanation on how to determine).
Question 2 (1 point): The atom of element X has a total of 40 particles (protons, electrons, neutrons). The total of charged particles is 12 more than the total of uncharged particles.
Calculate the number of each type of particle (protons, electrons, neutrons) in the atom X.
Question 3 (1 point): Given the following elements: Li (Z = 3), O (Z = 8), F (Z = 9), Na (Z = 11).
Arrange the elements in increasing order of atomic radius and provide a brief explanation of how they are arranged.
ANSWERS:
Part 1: Multiple Choice (7 points)
| 1-D | 2-C | 3-A | 4-B | 5-C | 6-A | 7-D | 8-A | 9-A | 10-D | 11-D |
| 12-C | 13-C | 14-C | 14-C | 15-A | 16-C | 17-D | 18-B | 19-A | 20-B | 21-D |
| 22-B | 23-D | 24-B | 25-B | 26-C | 27-D | 28-A |
Part 2: Essay (3 points)
Question 1:

Question 2:

Question 3:
We have:
+ Li, O, F are all in the same period 2 in the periodic table. In a period, as the nuclear charge increases, the atomic radius decreases ⇒ atomic radius:
F < O < Li (1)
+ Li and Na are both in group IA. In a group, as the nuclear charge increases, the atomic radius increases ⇒ atomic radius: Li < Na (2)
Thus, the sequence of elements ordered by increasing atomic radius is:
F, O, Li, Na.
Note: The above 1st mid-semester question papers of 10th-grade Chemistry and answers thereof are for reference only!
What are the 1st mid-semester question papers of 10th-grade Chemistry and answers thereof? What is the teaching equipment for 10th-grade Chemistry in Vietnam? (Image from the Internet)
What is the teaching equipment for 10th-grade Chemistry in Vietnam?
Under Section 8 in the General Education Program for Chemistry issued with Circular 32/2018/TT-BGDDT, the teaching equipment for 10th-grade Chemistry in Vietnam is specifically regulated as follows:
(1) The Chemistry teaching equipment set includes:
- Equipment for demonstrations and proofs
+ Periodic table of chemical elements; solubility table of salts and hydroxides; electron configuration table of metals/transition metal ions in the first series; color table of some transition metal compounds.
+ Pictures introducing the geometry of some complex compounds, Cu2+ salts in aqueous solvents; structure of some biological complexes like heme B, chlorophyll, vitamin B12, and used in medicine like cisplatin, carboplatin,...; 3R symbol; aluminum recycling; silicate industry; production of cement, ceramics and pottery for industry and handicrafts. Diagrams of distillation, processing, and applications of oil. Pictures about the application of alkane, alkene, alkadiene, and arene in practice; applications of halogen derivatives; alcohols and phenols in practice; roles of amino acids, roles of glucose, starch in life.
+ Models/sets for assembling hollow or compact molecular shapes of some alkanes; benzene, halogen derivatives, ethylic alcohol (ethyl alcohol), and phenol; amines, amino acids, peptides, and proteins.
+ Electronic learning materials:
Software: software for calculations; virtual experiment software.
Videos of harmful, dangerous explosive experiments, complex experiments,... such as experiments with chlorine, bromine,... alkali metals, alkaline earth metals interacting with water,...
(2) Equipment for practical use
- Analysis and measurement tools: electrolysis set for copper (2) sulfate solution and sodium chloride solution; conductivity tester; handheld pH meters;...
- There must be sufficient equipment, tools, and chemicals according to the minimum teaching equipment list prescribed by the Ministry of Education and Training.
What are the duties of 10th-grade students in Vietnam?
Under Article 34 of the lower secondary school, upper secondary school and multi-level school charter issued with Circular 32/2020/TT-BGDDT, the duties of 10th-grade students in Vietnam include:
- Learn and train according to curricula and education plans of their schools.
- Respect their parents, officials, teachers and staff of their schools, and those older than them; maintain solidarity and mutual support in learning and training; conform to the charter and rules of their schools; and abide by the law.
- Take exercises and maintain personal hygiene.
- Participate in group activities of their schools and classes, Ho Chi Minh Young Pioneer Organization and Ho Chi Minh Communist Youth Union; help their families, join physical and social activities and environmental protection activities, and maintain traffic order and safety.
- Protect school and public property; contribute to fostering, preservation and enhancement of school traditions.

