Vietnam: What are the newest 2nd mid-semester question papers and answers for 12th-grade Chemistry in the 2024-2025 school year?
What are the newest 2nd mid-semester question papers and answers for 12th-grade Chemistry in the 2024-2025 school year?
Students may refer to the following newest 2nd mid-semester question papers and answers for 12th-grade Chemistry in the 2024-2025 school year:
Department of Education and Training .....
2nd mid-semester question papers for 12th-grade Chemistry
Subject: 12th-grade Chemistry
Time: 45 minutes
Given the atomic mass of some elements: H = 1, C =12, N = 14, O = 16, Na = 23, Mg = 24, S = 32, Cl = 35.5, K = 39, Ca = 40, Ba = 137, Cu = 64, Ag = 108.
Question 1: Which of the following chemical equations can only be performed by electrolysis?
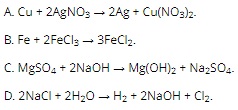
Question 2: Add 200 ml of 1M NaOH solution to 120 ml of 1M Ca(HCO3)2 solution, obtaining a grams of solid substance. The value of a is closest to which of the following values?
A. 11.8.
B. 23.5.
C. 19.7.
D. 9.7.
Question 3: Add 100 ml of 0.3M Ba(OH)2 solution to 50 ml of Al2(SO4)3 xM solution, resulting in 8.55 grams of precipitate. Then, add an additional 100 ml of 0.4M Ba(OH)2 solution to the reaction container, resulting in 18.8475 grams of precipitate. The value of x is
A. 0.45.
B. 0.5.
C. 0.3.
D. 0.6.
Question 4: Add 3.8 grams of a salt mixture M2CO3 and MHCO3 (M is an alkali metal) to an excess 2M H2SO4 solution, releasing 0.496 liters of gas (at standard conditions). M is
A. K.
B. Li.
C. Rb.
D. Na.
Question 5: Add m grams of a mixture G consisting of Na, Al, Fe to excess water, obtaining 4.48 liters of gas (at standard conditions). On the other hand, add m grams of the above G to a surplus NaOH solution, obtaining 7.84 liters of gas (at standard conditions) and solution X, solid Y. Completely dissolve Y in excess HNO3, obtaining 10.08 liters of NO2 (at standard conditions, sole reduction product). The value of m is
A. 23.9.
B. 47.8.
C. 16.1.
D. 32.2.
Question 6: Among the following statements:
(1) Li has a body-centered cubic lattice structure, used in the production of ultralight alloys.
(2) NaOH is used for manufacturing artificial fibers.
(3) CaO dissolves in water without releasing heat, dolomite ore has the formula MgCO3.CaCO3.
(4) Heating both temporary and permanent hard water results in a precipitate.
(5) Na2CO3 is applied to treat stomach ailments.
The incorrect statements are
A. (1), (2), (5).
B. (3), (4), (5).
C. (1), (2), (4).
D. (3), (5).
Question 7: The method of molten electrolysis is used to produce metals
A. of moderate activity like Fe, Zn.
B. of high activity like Ca, Na.
C. all metals like Cu, Na, Fe, Al.
D. of low activity like Ag, Au.
Question 8: Absorb 11.2 liters of CO2 (at standard conditions) into a solution containing only 25.9 grams of Ca(OH)2, obtaining a grams of solid Y. The value of a is
A. 15 grams.
B. 10 grams.
C. 20 grams.
D. 35 grams.
Question 9: Which of the following substances is used in: medicine (casting), sculpting,...?
A. CaSO4.
B. CaSO4.H2O.
C. CaSO4.2H2O.
D. BaCl2.H2O.
Question 10: Add 50ml of solution X containing: K2SO4 0.2M and Na2CO3 0.2M to an excess BaCl2 solution, obtaining a grams of solid Y. The value of a is closest to which of the following values?
A. 4.15 grams.
B. 2.93 grams.
C. 3.4 grams.
D. 3.9 grams.
Question 11: To reduce Fe3+ ions in Fe2(SO4)3 solution, which metal can be used
A. Mg.
B. Ba.
C. Na.
D. Ag.
Question 12: Which substance undergoes both thermal decomposition and possesses amphoteric properties
A. Al2O3.
B. CaCO3.
C. Na2CO3.
D. NaHCO3.
Question 13: Impure Ag containing impurities of Cu. Which reagent can be used to remove Cu to obtain pure Ag?
A. Excess HCl solution.
B. Concentrated, hot excess HNO3.
C. Limited AgNO3 solution.
D. Excess AgNO3 solution.
Question 14: When electrolyzing a CuSO4 solution (with inert electrodes), at the anode (A) occurs
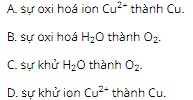
Question 15: Find the wrong statement?

Question 16: The substance used to soften all types of hard water is
A. Na2CO3 and CaO (quicklime).
B. Na2CO3 and Ca(OH)2 (slaked lime).
C. Na2CO3 and Na3PO4.
D. NaOH (caustic soda) and Ca(OH)2.
Question 17: Heating 200 grams of a mixture X consisting of Na2CO3 and NaHCO3 until the mass is constant, reducing the solid mass by 62 grams. The mass percentages of each substance in X are respectively
A. 37% and 63%.
B. 21% and 79%.
C. 42% and 58%.
D. 16% and 84%.
Question 18: A correct statement about the application of alkali metals and metal compounds of group IA is
A. NaOH is used to refine aluminum ore.
B. Li is used for manufacturing solar cells.
C. Cs is used to produce ultralight alloys.
D. Na2CO3 is used for soap making.
Question 19: Which of the following reactions does not occur under normal conditions?
A. CaO + H2O.
B. Al + NaOH solution.
C. Be + H2O.
D. Ba + H2O.
Question 20: Add 17.04 grams of a mixture X consisting of Ca, MgO, Na2O completely reacting with 360 ml of 2M HCl solution (just enough) obtaining solution Y. The mass (grams) of NaCl present in Y is
A. 4.68.
B. 8.775.
C. 15.21.
D. 14.04.
Question 21: Among the following metals: Na, Mg, Be, Fe, Ba, K, Sr, Ca. The number of metals that react vigorously with water under normal conditions is
A. 5.
B. 4.
C. 6.
D. 3.
Question 22: Which of the following substances is also known as slaked lime?
A. CaOCl2.
B. CaCO3.
C. CaO.
D. Ca(OH)2.
Question 23: Salt X when dissolved in water forms a neutral medium solution, X could be
A. Na2CO3.
B. NaCl.
C. Ca(HCO3)2.
D. KHSO4.
Question 24: Pass excess CO gas through mixture X consisting of: CuO, Fe2O3, Al2O3, MgO heated until completely reacted, obtaining solid mixture Y. Y consists of
A. Cu, Fe, Al, MgO.
B. Cu, Fe, Al2O3, MgO.
C. Cu, Fe2O3, Al2O3, MgO.
D. Cu, Fe, Al, Mg.
Question 25: Select the correct statement?
A. In industry, water bleaching is produced by electrolysis of NaOH solution.
B. In industry, Al is produced by electrolysis of molten AlCl3.
C. Carbonate salts of alkali and alkaline earth metals are decomposed by heat.
D. In industry, NaOH is produced by electrolysis of saturated brine with a diaphragm.
Question 26: The reaction explaining the erosion of limestone by rainwater (containing CO2) is
A. CaCO3 + CO2 + H2O ⇌ Ca(HCO3)2.
B. Ca(HCO3)2 → CaCO3 + CO2 + H2O.
C. CaCO3 + CO2 + H2O → Ca(HCO3)2.
D. CaO + H2O → Ca(OH)2.
Question 27: Heat mixture G consisting of: 7.2 grams of Mg and 4.8 grams of S in a sealed container (without air). After a while, obtaining solid X. Add X to an excess HCl solution obtaining gas mixture Y and solid Z. Completely combust Y and Z (with excess O2) then pass the entire combustion product slowly through excess limewater noting the change in solution mass is
A. decreased by 5.1 grams.
B. increased by 8.4 grams.
C. decreased by 3.0 grams.
D. increased by 15 grams.
Question 28: Add 240ml of 2M NaOH to 150 ml of 1M AlCl3 obtaining a grams of solid. The value of a is
A. 11.7.
B. 9.36.
C. 12.48.
D. 2.34.
Question 29: Which series of metals can be produced by electrolysis of solutions?
A. Ni, Cu, Fe, Na.
B. Fe, Cu, Mg, Ag.
C. Cu, Ag, Pb, Fe.
D. Mg, Fe, Zn, Na.
Question 30: Among alkali and alkaline earth metals, the metal with the lowest melting temperature is
A. Cs.
B. Li.
C. Ba.
D. Be.
ANSWERS AND DETAILED SOLUTIONS
Question 1. D
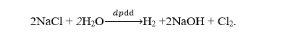
Question 2. A

Question 3. A

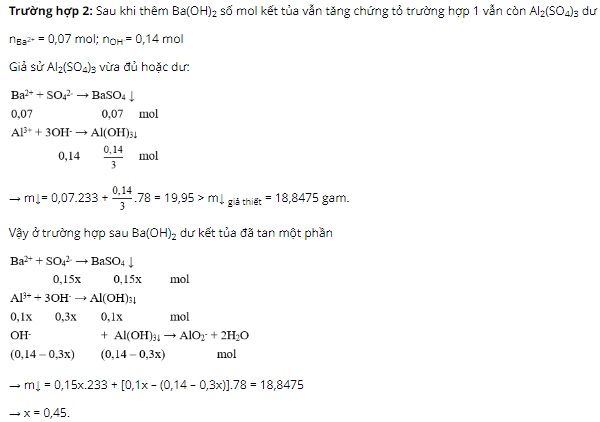
Question 4. C

Question 5. C

Question 6. D
(3) is incorrect because CaO dissolves in water with the release of significant heat.
(5) is incorrect because NaHCO3 is used to treat excessive stomach acid.
Question 7. B
The molten electrolysis method is used to produce metals of high activity such as Na, Ca.
Question 8. C
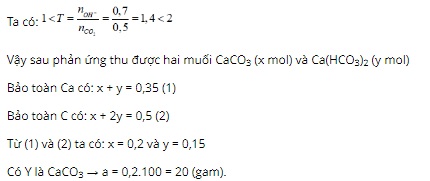
Question 9. B
Nitered gypsum: CaSO4.H2O is used in medicine (casting), sculpting, etc.
Question 10. A
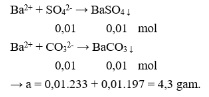
Question 11. A
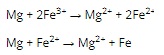
Question 12. D
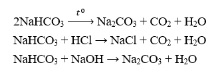
Question 13. D

Question 14. B
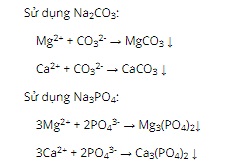
Question 15. B
Using just enough lime softens only temporary hard water.
Question 16. C
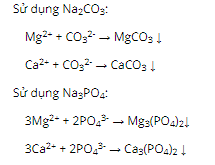
Question 17. D
Question 18. A
B is incorrect because Cs is used for manufacturing solar cells.
C is incorrect because Li is used for making ultralight alloys.
D is incorrect because NaOH is used for soap making.
Question 19. C
Be does not react with water under normal conditions.
Question 20. D
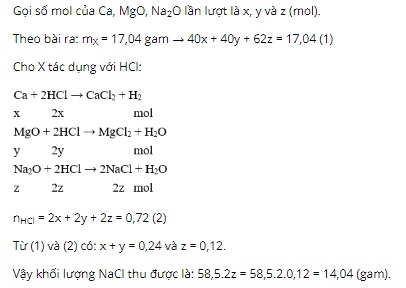
Question 21. A
Metals that react vigorously with water under normal conditions include: Na, Ba, K, Sr, Ca.
Question 22. D
Slaked lime: Ca(OH)2.
Question 23. B
NaCl is a salt formed from a strong metal and a strong acid radical, so it creates a neutral medium solution when dissolved in water.
Question 24. B

Question 25. D
A is incorrect because, in industry, water bleach is produced by electrolysis of NaCl solution without a diaphragm.
B is incorrect because, in industry, Al is produced by electrolysis of molten Al2O3.
C is incorrect because carbonate salts of alkali metals are not decomposed by heat.
Question 26. C

Question 27. C

Question 28. B

Question 29. C
The solution electrolysis method is used to produce metals with moderate and weak reducing properties.
A, B, and D are incorrect because Na, Mg are not produced by the solution electrolysis method.
Question 30. A
Among alkali metals and alkaline earth metals, Cs has the lowest melting point (29°C).
Note: Information is for reference purposes only!

What are the newest 2nd mid-semester question papers and answers for 12th-grade Chemistry in the 2024-2025 school year? (Image from the Internet)
What are the requirements regarding the education content for 12th-grade Chemistry in Vietnam?
According to Article 30 of the Education Law 2019 regulating the requirements regarding the education content for 12th-grade Chemistry in Vietnam:
Requirements on contents and methods of general education
1. The contents of general education must ensure the popular, basic, comprehensive, career-orienting, and systematic characteristics; linking with the realities of life, appropriate to the psycho-physiological characteristics of students, meeting the objectives of education at each level.
2. Requirements on the contents of general education at each level are regulated as follows:
a) Primary education must guarantee students the foundation for comprehensive development of physical and emotional health, social skills, simple and necessary knowledge about nature, society and human being; with social moral awareness; with basic skills in listening, reading, speaking, writing and calculating; with habits of physical exercise and hygiene; and with initial understanding of singing, dancing, music and arts;
b) Lower secondary education shall consolidate and develop the contents learned in primary education, guarantee students the basic general knowledge in Vietnamese, mathematics, national history, other knowledge in social sciences, natural sciences, law, informatics, foreign languages; with introductory understanding on techniques and career-orientation.
c) Upper secondary education shall consolidate and develop the contents learned in lower secondary education, complete the contents of general education. Besides guaranteeing the general, basic, comprehensive and career-orienting knowledge for all students, there shall be advanced teaching in some subjects to develop the students' abilities and satisfy their needs.
3. The methods of general education are to promote the activeness, consciousness, initiatives and creativeness of students; to be appropriate to the characteristics of each subject, class and student; to nurture the methods of self-study, the joy of learning, the ability to work in team, the ability of independent thinking; to develop comprehensively in dignity and ability; to enhance the application of information technology and communications to education.
Thus, under the above regulations, the education content for 12th-grade Chemistry in Vietnam shall consolidate and develop the contents learned in lower secondary education, complete the contents of general education. Besides guaranteeing the general, basic, comprehensive and career-orienting knowledge for all students, there shall be advanced teaching in some subjects to develop the students' abilities and satisfy their needs.
According to the applicable general education program, how does Chemistry benefit students in Vietnam?
Under Section 1 of the General Education Program for Chemistry issued with Circular 32/2018/TT-BGDDT:
Characteristics of the Subject
Chemistry is a science in the field of natural sciences, studying the composition, structure, properties, and transformations of elements and compounds.
Chemistry tightly combines theory and experiment, serving as a bridge among other natural sciences like physics, biology, medicine, and geology. Advances in the field of chemistry are closely tied to new discoveries in the fields of biology, medicine, and physics. Chemistry plays a crucial role in life, production, contributing to economic and social development. Achievements in chemistry are applied in fields like materials, energy, medicine, biotechnology, agriculture, forestry, and fisheries, among many others.
In the general education program, Chemistry is a subject within the group of natural sciences at the upper secondary level, chosen by students based on their career orientation, interests, and abilities. Chemistry helps students acquire core knowledge of chemistry and apply this knowledge in life, while also interrelating with multiple educational fields. Alongside Mathematics, Physics, Biology, Informatics, and Technology, Chemistry contributes to promoting STEM education, a trend valued in many countries worldwide.
The content of Chemistry is designed into topics that ensure the consolidation of content strands, development of knowledge and practical skills formed at the lower educational level, while also helping students gain deeper understanding of the foundational knowledge of chemistry, to serve as a basis for studying, working, and researching.
...
Thus, according to the new program characteristics, Chemistry helps students acquire core knowledge of chemistry and apply this knowledge in life, while also interrelating with multiple educational fields.

